All published articles of this journal are available on ScienceDirect.
Real-world Effectiveness and Tolerability of Apremilast in Psoriatic Arthritis in Germany: Results from LAPIS-PsA
Abstract
Background
Apremilast is approved for psoriasis and psoriatic arthritis (PsA). Although clinical trials demonstrated efficacy and tolerability of apremilast, real-world data for PsA are lacking.
Objective
Assess the real-world effectiveness and tolerability of apremilast in German clinical practice.
Methods
The multicenter, prospective, non-interventional LAPIS-PsA study (conducted from February 2016 to July 2018) enrolled patients diagnosed with moderate to severe PsA (Physician’s Global Assessment [PhGA] ≥2) and insufficient response or intolerance to ≥1 disease-modifying antirheumatic drug who were initiating apremilast in German clinical practice. Outcomes included, but were not limited to, PhGA improvement ≥1 (scale of 0–4) at ~7 months (primary); PhGA improvement ≥1, Patient Global Assessment (PtGA) improvement ≥1, pain visual analog scale (VAS), pruritus VAS, and the 9-item Psoriatic Arthritis Impact of Disease (PsAID-9) over ~13 months (secondary); and minimal disease activity (MDA) over ~13 months (exploratory).
Results
A total of 418 patients were analyzed; mean±SD age: 54.9±11.0 years, 59.2% female, 40.8% male. Most (75.6%) patients achieved a PhGA ≥1-point improvement at ~7 months, with responses maintained at ~13 months (72.5%). Over half (58.7%) achieved a PtGA improvement ≥1-point; over one-third (41.5%) achieved MDA at ~13 months. Mean±SD pain and pruritus VAS decreased from 52.1±23.1 and 36.7±30.7 at baseline to 32.6±23.4 and 19.6±21.9 at ~13 months, respectively. Mean (SD) PsAID-9 total score decreased from 5.3±2.1 at baseline to 3.3±2.0 at ~13 months.
Conclusion
Patients with PsA treated with apremilast in German clinical practice experienced improvements in patient- and physician-reported measures of disease activity over 13 months of treatment.
1. INTRODUCTION
Psoriatic arthritis (PsA) occurs in approximately 30% of patients with psoriasis [1]. PsA can present differently from one patient to another, with signs ranging from mild symptoms to severe joint erosion. Joint erosion is progressive in nature and can result in functional impairment and disability and severe impairment in quality of life [1, 2]. Peripheral arthritis and extra-articular manifestations of PsA, such as enthesitis, dactylitis, and nail pitting, also contribute to severity [1, 3]. Additionally, PsA is associated with many comorbidities, particularly cardiometabolic disease [4-6]. As a result of this burden, patients report that PsA negatively impacts their quality of life, functioning, and ability to work [7-9].
Apremilast is an oral phosphodiesterase 4 (PDE4) inhibitor approved in several countries around the world. Apremilast was approved in the United States in 2014 for active PsA in adults and in Europe in 2015 for active PsA in adults with an inadequate response or intolerance to prior disease-modifying antirheumatic drugs (DMARDs) [10]. Patients can be treated with apremilast alone or in combination with conventional systemic DMARDS. According to the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations, PDE4 inhibitors are strongly recommended for peripheral arthritis in both DMARD-naive patients (off-label) and patients with an inadequate response to DMARDs [5]. The GRAPPA recommendations also note PDE4 inhibitors are strongly recommended for PsA disease manifestations such as psoriasis, enthesitis, dactylitis, and nail disease. The 2023 update of the European Alliance of Associations for Rheumatology guidelines advise that apremilast should be used when conventional systemic DMARDs have failed and both biologic DMARDs and Janus kinase inhibitors are inappropriate, noting that apremilast may have the most clinical value in patients with mild disease [11].
In the phase 3 PALACE clinical trials, apremilast demonstrated significantly greater improvements in PsA disease severity, skin involvement, enthesitis, dactylitis, and physical function versus placebo [12-15]. However, clinical trial populations do not reflect the heterogenous patient populations treated in clinical practice, and evidence from routine clinical practice is needed in addition to clinical trial data. There are currently limited studies of apremilast use in patients with PsA in clinical practice. LAPIS-PsO (ClinicalTrials.gov identifier NCT02626793) previously reported the effectiveness and tolerability of apremilast for the treatment of psoriasis in German clinical practice [16]. Here, we report data from LAPIS-PsA (ClinicalTrials.gov identifier NCT03106051), which evaluated the effectiveness and tolerability of apremilast for the treatment of PsA in German clinical practice.
2. MATERIALS AND METHODS
2.1. Study Design
LAPIS-PsA was a multicenter, prospective, observational study of patients with PsA initiating apremilast (30 mg twice daily [BID]) under routine care in Germany (Fig. 1). Patients were treated as per regional policies or local legislation/admission conditions and according to the summary of product characteristics (SmPC) [10]. Enrollment began in February 2016, and the last visit occurred in August 2020. Study visits occurred as per clinical practice; no specific timing of study visits was mandated. Baseline (visit 0) was defined as apremilast initiation. Visit 1 was optional and occurred 4–6 weeks after baseline (month ~1); visits 2 to 5 were approximately 4, 7, 10 and 13 months after baseline, respectively. The study was originally planned for 13 months (visits 1–5) but was extended to 25 months (2 additional visits) while the study was ongoing to capture a longer follow-up period.
This study was conducted in accordance with the Declaration of Helsinki and the protocol approved by either regional or institutional ethics boards. Informed written consent was obtained from each patient prior to any study-related procedure.
2.2. Eligibility Criteria
This study included patients ≥18 years of age who met Classification Criteria for Psoriatic Arthritis (CASPAR), with a diagnosis of at least moderate PsA disease activity (Physician’s Global Assessment [PhGA] ≥2) and insufficient response or intolerance to ≥1 prior DMARD. Patients were excluded if they were pregnant or breastfeeding, had
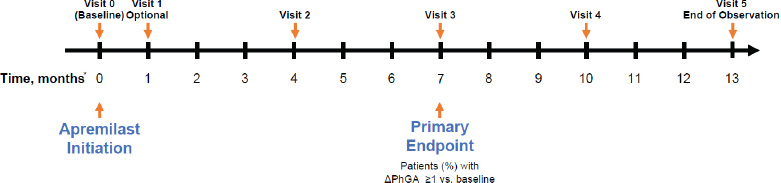
LAPIS-PsA study design.
*Timing is approximate, and visits occurred according to clinical practice; no strict visit schedule was mandated in the protocol.
PhGA, Physician’s Global Assessment.
a hypersensitivity to apremilast or one of the other ingredients in the film tablets, or other criteria according to the prescribing information [10]. The decision to treat with apremilast was made independently by the attending physician before the patient was included in the study.
2.4. Outcomes
Due to limited patient numbers during the last 2 visits, only efficacy data through month ~13 (visits 1–5; the original planned end of the study) are presented. The study measured changes in disease activity as indicators of apremilast's effectiveness (Table S1). The tolerability of apremilast was also assessed. The primary outcome was a reduction in the score (i.e., improvement) on the PhGA by at least 1 point (on a scale of 0 [no symptoms] to 4 [very strong symptoms]) ~7 months after apremilast initiation (i.e., at month ~7 relative to baseline).
All secondary outcomes were assessed at each visit through month ~13, unless otherwise stated, and included a reduction in the score (i.e., improvement) on the Patient Global Assessment (PtGA) by at least 1 point (on a scale of 0 [excellent] to 4 [very bad]); a reduction in score on the PhGA by at least 1 point; mean values and percent changes from baseline on the tender joint count (TJC-68; on a scale of 0–68, with higher values indicating more tenderness) and swollen joint count (SJC-66; on a scale of 0–66, with higher values indicating more swelling); percentage of patients achieving resolution of dactylitis (ie, dactylitis count=0, on a scale of 0–20); mean Leeds Enthesitis Index (LEI) score (on a scale of 0 [no tender entheses] to 6 [6 tender entheses]); percentage body surface area (BSA) involvement; and mean values and change from baseline in patient-reported visual analog scales (VAS) for pain and pruritus (on a scale of 0–100, with higher values indicating worse health).
Additional secondary endpoints were scores on the 9-item Psoriatic Arthritis Impact of Disease (PsAID-9) at months ~1, ~4, ~7, and ~13; Patient Preference Questionnaire (PPQ) at months ~7 and ~13; and the Hannover Functional Ability Questionnaire (FFbH) at months ~1, ~4, ~7, and ~13. The PsAID-9 is a 9-item, self-administered questionnaire that measures the impact of PsA from the perspective of the patient and consists of both physical and psychological domains. Scores range from 0 (best state) to 10 (worst state), with a patient-acceptable symptom state (PASS) defined as a score ≤4 [17]. The PPQ is a 5-item questionnaire that asks patients whether they prefer the current treatment (apremilast) to previous systemic therapies in certain respects, such as effectiveness, ease of use, and side effects. The FFbH is a short, 12-item, self-administered questionnaire that measures functional limitations in daily activities for patients with rheumatic diseases, with a composite score ranging from 0 to 100% normal functioning capacity.
Minimal disease activity (MDA) was assessed as an exploratory outcome at months ~1, ~4, ~7, and ~13. MDA was calculated as a composite score comprising 7 criteria: TJC ≤1, SJC ≤1, BSA ≤3%, pain VAS ≤15, Health Assessment Questionnaire (HAQ) ≤0.5, PtGA ≤20, and LEI ≤1, with patients considered MDA responders if they met 5 of the 7 criteria [18].
The proportion of patients in each PhGA and PtGA category and mean score on the HAQ-Disability Index (HAQ-DI; on a scale of 0 [without any difficulty] to 3 [unable to do]) were also assessed at months ~1, ~4, ~7, ~10, and ~13 as a post hoc analysis. Safety outcomes included adverse events (AEs) and were assessed through month ~25 (visit 7).
2.5. Statistical Analysis
All outcomes were summarized using descriptive statistics. Safety outcomes were analyzed using the safety analysis set (SAS), which included all patients who received at least one dose of apremilast in line with regional policies and the SmPC. All other outcomes were analyzed using the full analysis set (FAS), which included all patients who received ≥1 dose of apremilast and had a baseline PhGA score and ≥1 post-baseline visit. The primary analysis for the primary outcome used the last observation carried forward (LOCF) for missing data. All other analyses were as observed. Mean HAQ scores were calculated from FFbH scores according to the formula HAQ = 3.16 - 0.028*FFbH [19]. A subgroup analysis was conducted to assess PhGA ≥1-point improvement response rates, PtGA ≥1-point improvement response rates, PsAID-9 scores, and MDA response rates in patients who had previously received biologic therapy and patients who had not previously received biologic therapy.
3. RESULTS
3.1. Population Characteristics
Patient dispositions are summarized in Fig. (2), and baseline patient characteristics are summarized in Table 1. Of 549 patients enrolled, 418 were included in the FAS and 484 were included in the SAS. Among patients in the FAS, the mean (SD) age was 54.9 (11.0) years, with 59.2% female and 40.8% male. The mean time from diagnosis of PsA to apremilast initiation was 17.5 years, and three-quarters (75.6%) of patients in the FAS had pre-existing comorbidities. Cardiovascular disease and obesity were the most common comorbidities, reported in half and one-fifth of patients, respectively. One-quarter of patients (25.8%) had previously received biologics for PsA, and 8.1% had previously received biologics for psoriasis. Prior therapies for PsA and psoriasis are shown in Table S2. More than half (223/418 [53.3%]) of the patients in the FAS and 47.7% (231/484) of those in the SAS completed visit 5 (month ~13). A total of 208 patients discontinued the study (Fig. 2), with the most common reasons being lack of effectiveness and adverse events (111/484 [22.9%] and 86/484 [17.8%] patients, respectively, in the SAS).
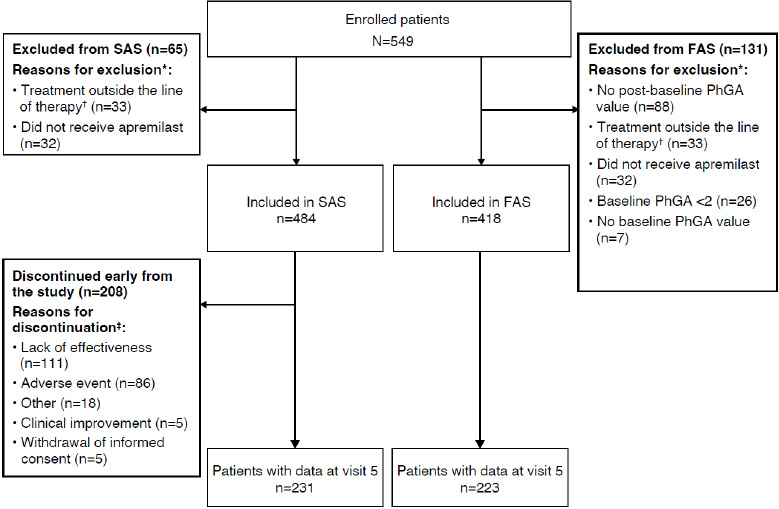
Patient disposition.
The SAS included all patients who received ≥1 dose of apremilast. The FAS included all patients who received ≥1 dose of apremilast and had a baseline PhGA score ≥2 and ≥1 post-baseline visit.
*A patient may have >1 reason for exclusion.
†Patients taking apremilast outside of the line of therapy are defined as patients for whom the use of at least one systemic, disease-modifying drug for PsA and plaque psoriasis was not documented in the case report form.
‡A patient may have >1 reason for discontinuation.
FAS, full analysis set; PhGA, Physician’s Global Assessment; PsA, psoriatic arthritis; SAS, safety analysis set.
| Characteristic |
Full Analysis Population N=418 |
|---|---|
| Female, n (%) | 244 (59.2) |
| Age at inclusion, mean (SD), years | 54.9 (11.0) |
| BMI, mean (SD), kg/m2 | 29.6 (5.8) |
| Patients with ≥1 comorbidity, n (%) | 316 (75.6) |
| Cardiovascular disease | 214 (51.2) |
| Hypertension | 203 (48.6) |
| Coronary heart disease | 29 (6.9) |
| Other | 16 (3.8) |
| Cerebrovascular disease | 3 (0.7) |
| Arterial disease | 2 (0.5) |
| Obesity | 88 (21.1) |
| Diabetes mellitus | 61 (14.6) |
| Hyperlipoproteinemia | 58 (13.9) |
| Depression | 48 (11.5) |
| Hyperuricemia | 37 (8.9) |
| Renal disease | 25 (6.0) |
| Other autoimmune disease | 21 (5.0) |
| Liver disease | 15 (3.6) |
| Infectious disease | 10 (2.4) |
| Inflammatory eye disease | 9 (2.2) |
| Malignant neoplasia | 9 (2.2) |
| Other comorbidities | 145 (34.7) |
| Previous PsA therapies, n (%) | |
| Conventional systemic | 397 (95.0) |
| Biologic | 108 (25.8) |
| Previous psoriasis therapies, n (%) | |
| Conventional systemic | 155 (37.1) |
| Fumaric acid esters* | 27 (6.5) |
| Biologic | 34 (8.1) |
| Topical therapies | 90 (21.5) |
| Phototherapy | 38 (9.1) |
| PhGA ≥3, n (%) | 179 (42.8) |
| PtGA ≥3, n (%) | 231 (55.7) |
| PsAID-9, mean (SD) | 5.3 (2.1) |
| MDA, n (%)† | 13 (3.8) |
3.2. Global Disease Assessment
Three-quarters of patients in the FAS achieved the primary outcome of PhGA ≥1-point improvement at month ~7 (LOCF; Fig. 3A), with slightly higher response rates in biologic-naive (79.5% [241/303] by LOCF) versus biologic-experienced (64.6% [73/113] by LOCF) patients (Fig. S1A); response rates for data as observed are shown in (Fig. S1B). Rates of PhGA ≥1-point improvement were maintained at month ~13 (72.5%). PtGA ≥1-point improvement was achieved by 57.2% at month ~7 (Fig. 3B), with similar response rates in biologic-naive (125/213 [58.7%]) and biologic-experienced (36/68 [52.9%]) patients (Fig. S1C). This increased to 58.7% by month ~13.
The percentage of patients with no or minimal symptoms (PhGA score 0 or 1) increased over time, from 0% at baseline to 77.7% by month ~13 (Fig. 3C). The percentage of patients rating their disease as excellent or good (PtGA score 0 or 1) also increased over time, from 7.4% at baseline to 43.1% by month ~13 (Fig. 3D).
3.3. Joint and Skin Involvement
Mean (95% CI) TJC decreased from 11.3 (10.1, 12.6) at baseline to 3.3 (2.1, 4.5) at month ~13 (mean [95% CI] percent change, −61.0% [−75.6, −46.5]); mean (95% CI) SJC decreased from 4.6 (4.1, 5.0) at baseline to 0.8 (0.5, 1.1) at month ~13 (mean [95% CI] percent change: −77.4% [−87.1, −67.6]) (Table 2, Fig. 4).
| Baseline | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
|---|---|---|---|---|---|---|
| TJC, n, mean (95% CI) |
418 11.3 (10.1, 12.6) |
322 7.9 (6.4, 9.3) |
357 5.6 (4.4, 6.8) |
298 4.3 (3.0, 5.6) |
250 3.7 (2.4, 4.9) |
223 3.3 (2.1, 4.5) |
| SJC, n, mean (95% CI) |
417 4.6 (4.1, 5.0) |
321 2.5 (2.1, 2.9) |
357 1.8 (1.4, 2.2) |
298 0.8 (0.6, 1.0) |
250 1.1 (0.7, 1.5) |
223 0.8 (0.5, 1.1) |
| Dactylitis count = 0 (in patients with baseline dactylitis count >0), n/N (%) | 0/95 (0.0) |
33/66 (50.0) |
57/79 (72.2) |
51/67 (76.1) |
46/56 (82.1) |
41/46 (89.1) |
| LEI total score (in patients with baseline LEI >0), n, mean (SD) |
188 2.9 (1.7) |
147 1.7 (2.0) |
147 1.4 (1.9) |
117 1.3 (1.9) |
102 1.2 (1.9) |
84 1.0 (1.7) |
| LEI = 0 (in patients with baseline LEI >0), n/N (%) | 0/188 (0.0) |
57/147 (38.8) |
75/147 (51.0) |
60/117 (51.3) |
59/102 (57.8) |
50/84 (59.5) |
| Psoriasis-involved % BSA, n, mean (SD) | 391 9.9 (14.3) |
304 7.7 (12.7) |
321 4.7 (8.5) |
267 3.1 (5.2) |
219 2.9 (6.7) |
196 2.4 (4.3) |
| Pain VAS (0–100 mm), n, mean (95% CI) |
414 52.1 (49.9, 54.4) |
309 44.9 (42.2, 47.7) |
342 39.3 (36.6, 41.9) |
282 35.5 (32.6, 38.4) |
238 36.0 (32.9, 39.2) |
210 32.6 (29.4, 35.7) |
| Pruritus VAS (0–100 mm), n, mean (95% CI) |
413 36.7 (33.7, 39.6) |
305 26.8 (23.8, 29.8) |
336 23.1 (20.4, 25.8) |
282 22.1 (19.4, 24.7) |
237 23.7 (20.5, 26.9) |
207 19.6 (16.6, 22.6) |
The proportions of patients reporting dactylitis decreased over time (through month ~13) (Table 2, Fig. S2A). Among 95 patients reporting a dactylitis count >0 at baseline who continued apremilast through ~month 13, most (41/46, 89.1%) achieved a dactylitis count of 0 at ~month 13. Mean LEI also decreased over time (Table 2, Fig. S2B). Among 188 patients reporting LEI >0 at baseline, over half (50/84 [59.5%]) of those who continued apremilast through ~month 13 reported an LEI of 0 at month ~13; mean LEI decreased from 2.9 at baseline to 1.0 at ~month 13 (mean [SD] change: −2.1 [2.1]). Improvements were also observed in skin involvement (Table 2 and Fig. S3). Mean (SD) BSA fell from 9.9% (14.3) at baseline to 2.4% (4.3) at month ~13.
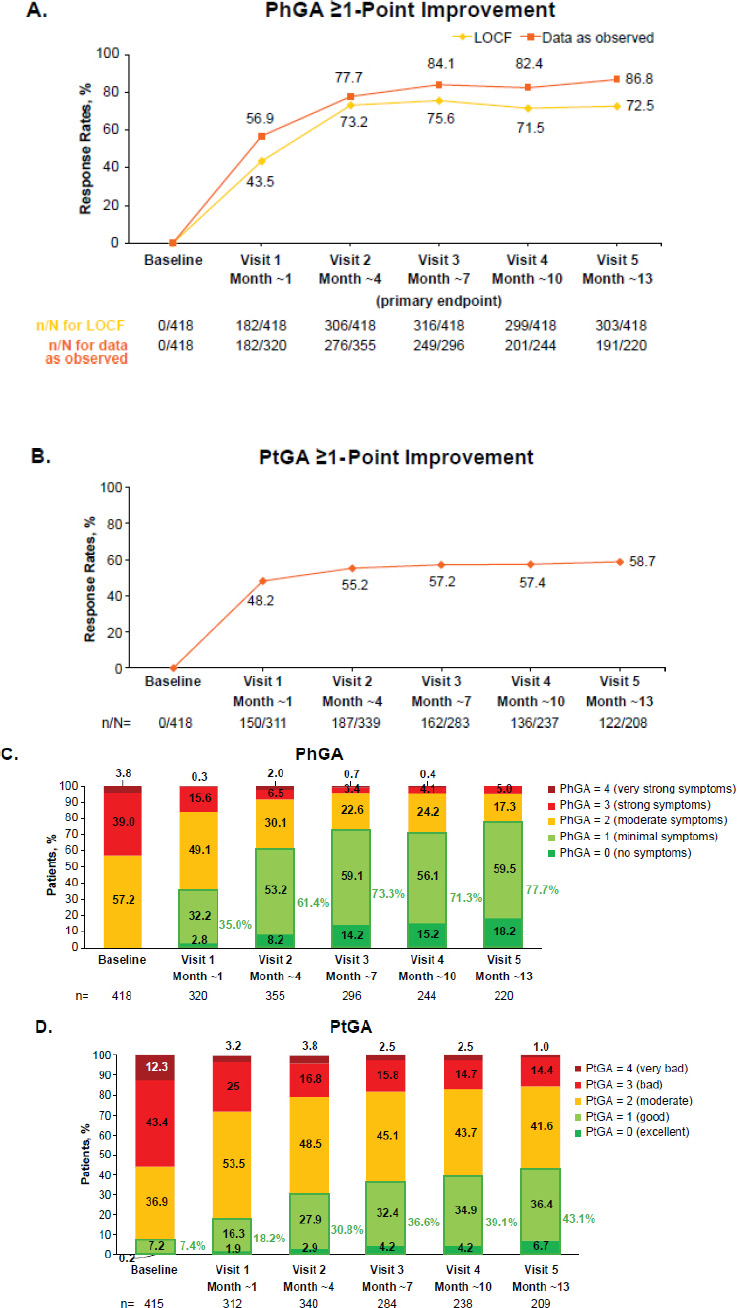
(A) PhGA ≥1-point improvement,* (B) PtGA ≥1-point improvement,† and change in (C) PhGA over time‡ and (D) PtGA over time,‡ in patients with PsA receiving apremilast in German clinical practice.
*Data are from the FAS (N=418). For data as observed, n/N = number of patients who achieved response/number of patients with available data.
†Data are from the FAS (N=418). Data as observed.
‡Data as observed. Includes patients with PhGA or PtGA values available at each visit.
FAS, full analysis set; LOCF, last observation carried forward; PhGA, Physician’s Global Assessment; PsA, psoriatic arthritis; PtGA, Patient Global Assessment.
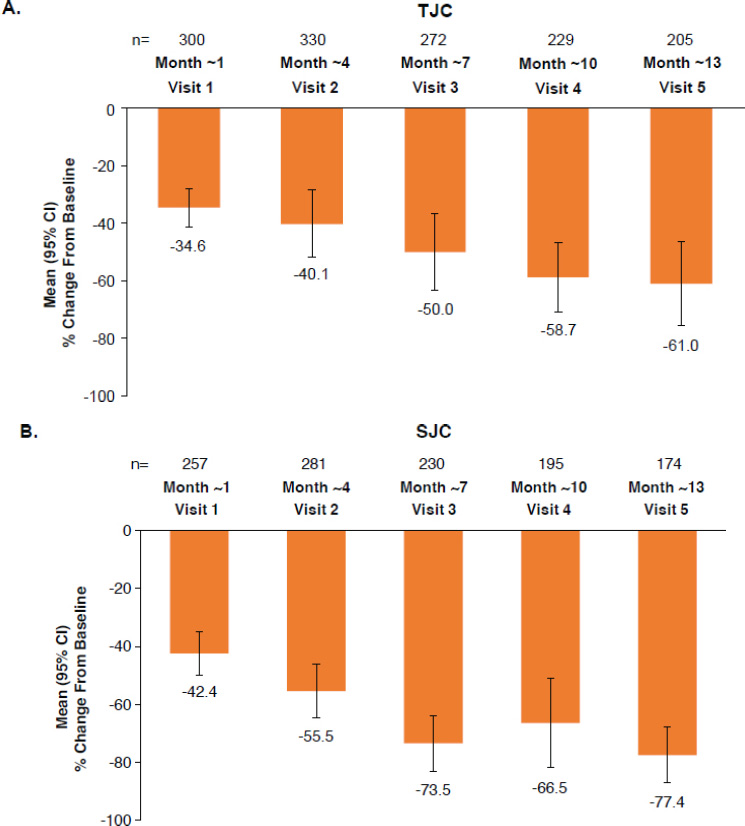
Percent change from baseline in (A) TJC and (B) SJC over time in patients with PsA receiving apremilast in German clinical practice.
Full analysis set. Data as observed.
CI, confidence interval; PsA, psoriatic arthritis; SJC, swollen joint count; TJC, tender joint count.
3.4. Patient-reported outcomes
Mean (95% CI) patient-reported VAS for pain decreased from 52.1 (49.9, 54.4) mm at baseline to 32.6 (29.4, 35.7) mm at month ~13 (mean [SD] change: −16.3 mm [−20.0, −12.7]); mean (95% CI) pruritus VAS decreased from 36.7 (33.7, 39.6) mm at baseline to 19.6 (16.6, 22.6) mm at month ~13 (mean [95% CI] change: −14.5 mm [−18.2, −10.9]) (Table 2, Fig. S4). Mean (SD) PsAID-9 score decreased from 5.3 (2.1) at baseline to 3.5 (2.1) at month ~7 and to 3.3 (2.0) at month ~13 (Table 2, Fig. 5A). Mean PsAID-9 scores reached the threshold for PASS (≤4) from month ~4 onward. Greater decreases in PsAID-9 scores were observed in biologic-naive versus biologic-experienced patients (mean [SD] change at ~month 13: −1.7 [2.1] vs. −1.4 [1.7]) (Table 2, Fig. 5B).
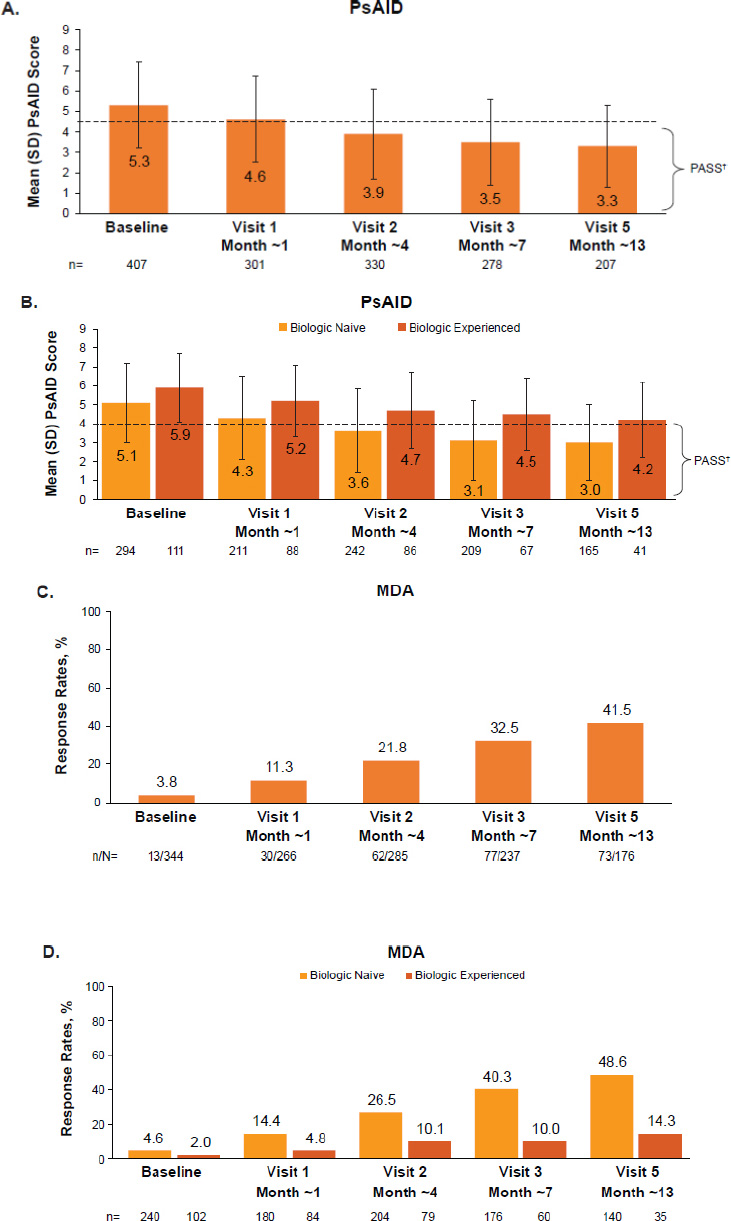
(A) PsAID-9 scores over time, (B) PsAID-9 scores over time summarized by use of prior biologics, (C) MDA* achievement over time, and (D) MDA* achievement summarized by use of prior biologics in patients with PsA receiving apremilast in German clinical practice.
Full analysis set. Data as observed.
*As per clinical practice, there was no PsAID-9 or MDA assessment scheduled at visit 4 (~10 months after baseline).
†PASS is defined as PsAID-9 ≤4, indicated by the dotted line.
MDA, minimal disease activity; PASS, patient-acceptable symptom state; PsA, psoriatic arthritis; PsAID-9, 9-item Psoriatic Arthritis Impact of Disease; SD, standard deviation.
|
Safety Analysis Population N=484 |
|
|---|---|
| ≥1 AE, n (%) | 288 (59.5) |
| ≥1 treatment-related AE, n (%) | 195 (40.3) |
| ≥1 treatment-related and at least moderately severe AE, n (%) | 134 (27.7) |
| AEs leading to treatment discontinuation, n (%) | 134 (27.7) |
| ≥1 SAE, n (%) | 45 (9.3) |
| ≥1 treatment-related SAE, n (%) | 23 (4.7) |
| Treatment-related AEs occurring in ≥2% of patients, n (%) | |
| Diarrhea | 56 (11.6) |
| Nausea | 35 (7.2) |
| Drug ineffective | 29 (6.0) |
| Psoriasis | 21 (4.3) |
| Headache | 20 (4.1) |
| Psoriasis arthropathica | 12 (2.5) |
| Vomiting | 12 (2.5) |
| Abdominal pain | 10 (2.1) |
As reported in the PPQ at months ~7 and ~13, more than 80% of patients preferred apremilast to their previous systemic therapies (Fig. S5). Patients reported that apremilast was more effective, more convenient in application, had fewer side effects, and was more tolerable than previous systemic therapies. As measured by the FFbH, patient functioning also improved, with 42.1% of patients who reported <80% normal functioning at baseline reporting 80–100% normal functioning by month ~13 (Fig. S6). Similar results were seen when functioning was measured by mean (SD) HAQ-DI, which decreased from 1.06 (0.53) at baseline to 0.82 (0.48) at ~month 13 (Table S3).
3.5. MDA
One-third of patients achieved MDA at month ~7, and 41.5% achieved MDA at month ~13 (Fig. 5C). MDA rates were higher among biologic-naive versus biologic-experienced patients (48.6% vs. 14.3% at month ~13, respectively) (Fig. 5D).
3.6. Safety
Over half (59.5%) of patients in the SAS reported at least one AE and 40.3% reported a treatment-related AE (Table 3); 9.3% reported at least one serious AE (SAE), and 5.0% reported at least one treatment-related SAE (Table 3, Table S4). The most common (>2%) treatment-related AEs were diarrhea (11.6%), nausea (7.2%), drug ineffective (6.0%), psoriasis (4.3%), headache (4.1%), PsA (2.5%), vomiting (2.5%), and abdominal pain (2.1%). The most common treatment-related SAEs were drug ineffective (n=7; 1.4%), PsA (n=5; 1.0%), diarrhea (n=3; 0.6%), and psoriasis (n=2; 0.4%). Treatment-related SAEs are described in Supplementary Results.
4. DISCUSSION
LAPIS-PsA was a large, multicenter, observational study of patients with PsA receiving apremilast in German clinical practice. In this study, patients experienced improved clinical and patient-reported outcomes. Approximately 7 months after apremilast initiation, 75.6% of patients (by LOCF) and 84.1% of patients (by data as observed) achieved a ≥1-point improvement in the PhGA, while 57.2% of patients reported a ≥1-point improvement in PtGA. Similarly, at month ~13, three-quarters (77.7%) of patients achieved a PhGA score of 0 or 1 (no or minimal symptoms) compared with 43.1% of patients who achieved a PtGA score of 0 or 1 (good or excellent). These differences may be due to a higher proportion of patients with a score of 3 or 4 at baseline for the PtGA than the PhGA. Several studies have reported differences in patient and physician perceptions of disease, with patients consistently rating their disease as more severe than their physicians. Fatigue, psychological factors, pain, and disability have been found to contribute to higher patient ratings, and SJC and TJC have been found to contribute to higher physician ratings [20, 21]. Nonetheless, many patients reported symptoms improving from very bad or bad to at least moderate over the 13-month period.
We observed improvements in multiple measures of disease activity during apremilast treatment. The mean PsAID-9 score reached the threshold for PASS by month ~4 and remained below this threshold thereafter. The proportion of patients achieving MDA increased throughout the study, from 3.8% at baseline to 32.5% at month ~7 and 41.5% at month ~13. Dactylitis and enthesitis were present in 22.7% and 45.0% of patients at baseline, respectively. Resolution of dactylitis symptoms was reported in 76.1% of patients at month ~7 and in 89.1% at month ~13. Enthesitis scores also decreased over time, and 59.5% of patients with LEI >0 at baseline reported an LEI score of 0 at month ~13. Joint and skin involvement, pain, and pruritus also decreased. With patients commonly reporting pruritus to be the most bothersome symptom of their disease, the decrease in pruritus is of particular clinical relevance [22, 23]. These improvements in clinical parameters were accompanied by improvements in functioning capacity as measured by FFbH. Among patients who were functioning at <80% of their normal capacity at baseline, 42.1% were reported to be functioning at 80–100% by month ~13. How physical function changes with apremilast treatment is a valuable insight from LAPIS-PsA. In addition, more than 80% of patients preferred apremilast over previous systemic therapies, with 75–80% reporting it to be more effective, more convenient, and better tolerated with fewer side effects. The safety profile was consistent with clinical trials and observational studies of apremilast [24-26], and no new safety signals were observed; common AEs were diarrhea and nausea, and the incidence of SAEs was low.
We observed little to no difference between the percentage of biologic-naive and biologic-experienced patients achieving a PhGA ≤1 and/or PtGA ≤1. However, compared with biologic-experienced patients, biologic-naive patients were more likely to achieve MDA and had lower PsAID-9 scores at baseline and months ~7 and ~13, suggesting biologic-naive patients may benefit more from apremilast therapy. In addition, biologic treatment is reserved for patients with greater disease severity, which may, in part, contribute to the differences we observed.
Mean SJC and TJC were low at baseline (4.6 and 11.3, respectively). Most clinical trials of apremilast in PsA enrolled patients with ≥3 swollen and ≥3 tender joints, with mean baseline SJC and TJC approximately 10 and 20, respectively, in the phase 3 PALACE and ACTIVE trials [12-15, 27]. Our data indicate that patients receiving apremilast in clinical practice in Germany have lower levels of joint involvement than observed in these clinical trials. Despite this, the FOREMOST trial is currently the only randomized placebo-controlled trial of apremilast in patients with more limited joint involvement [28]. Further analysis of apremilast use in patients with fewer affected joints may be warranted. Despite lower baseline TJC and SJC, the mean baseline pain VAS score in LAPIS-PsA (52.1) was similar to those reported in PALACE (52.6–61.2), and similar mean changes in pain were observed in the apremilast 30 mg BID groups (LAPIS-PsA: −12.0 at month ~4 and −14.8 at month ~7; PALACE: −9.8 to −14.8 at weeks 16 or 24) [12, 13, 15].
Comorbidity rates, particularly cardiometabolic diseases, were higher in our real-world population than in a pooled analysis of 15 apremilast clinical trials, which included five studies in patients with PsA. Specifically, this pooled analysis reported hypertension to be the most common comorbidity among patients with PsA, occurring in 35.6% of patients [24], with obesity and diabetes occurring in 12.3% and 5.8% of patients, respectively. In LAPIS-PsA, hypertension was reported in 48.6% of patients, obesity in 21.1%, and diabetes in 14.6%. This higher rate of comorbidities in clinical practice compared with randomized, controlled clinical trials highlights the value of real-world studies. Despite these comorbidities, the aforementioned pooled analysis reported low rates of major adverse cardiovascular events and thrombotic events (0.5% and 0.2%, respectively) with up to 5 years of apremilast treatment [24]. Thus, although patients in German clinical practice may have high rates of cardiometabolic comorbidities, apremilast is not expected to increase the risk of cardiovascular adverse events.
Although many studies have assessed the effectiveness of apremilast for psoriasis in clinical practice, few studies have evaluated PsA in this setting. APOLO and APPRAISE were multicenter, observational, prospective studies of apremilast in 106 patients with PsA in Belgium (NCT03096990) [25] and 102 patients with PsA in Canada (NCT03608657) [26], respectively. Similar to LAPIS-PsA (N=549), APPRAISE reported low baseline SJC and TJC (5.4 and 7.5, respectively), supporting our observation that patients receiving apremilast in real-world clinical practice have limited joint involvement. Similar decreases in SJC, TJC, and PsAID-9 score were observed in APOLO, APPRAISE, and LAPIS-PsA. In addition, consistent with our data, the rate of cardiometabolic comorbidities was high (49.0%) in APPRAISE. In APOLO, 40.7% of patients achieved PhGA=1 and 21.6% achieved PtGA =1 at month 6, similar to the results we observed at month ~7 (59.1% and 32.4%, respectively). Resolution of enthesitis and dactylitis was consistently observed in APOLO, APPRAISE, and LAPIS-PsA. Reported AEs were similar among LAPIS-PsA, APOLO, and APPRAISE, with the most common being diarrhea and nausea for all studies. Treatment-related AEs were reported in 40.3% of patients in LAPIS-PsA and 45.3% of patients in APOLO. SAEs were low in both LAPIS-PsA (9.3%) and APPRAISE (3.9%). Collectively, these studies report consistent effectiveness and tolerability for apremilast in the treatment of PsA across routine care settings.
As a real-world study of German clinical practice, LAPIS-PsA is limited by the loss of patients over time. The strengths of our study include an assessment of the real-world effectiveness of apremilast in routine clinical practice, a relatively large sample size, and a diverse collection of outcomes that provides a comprehensive assessment of changes in disease parameters from the perspectives of the attending physicians and patients. Moreover, LAPIS-PsA provides an evaluation of how patients who are eligible for apremilast in routine care will benefit from the treatment.
CONCLUSION
Patients with PsA treated with apremilast in clinical practice across Germany experienced improvements in both patient- and physician-reported outcomes that were maintained over ~13 months of treatment. These improvements were observed for the signs and symptoms of PsA, skin involvement, itch, and pain. The safety profile was consistent with the known safety profile of apremilast. In combination with results from clinical trials and other observational studies, our data support apremilast as an effective and well-tolerated treatment for PsA.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: study conception and design were contributed by JW, data collection was provided by JW and MF and Data Analysis or Interpretation was presented by FB, JW, MF, RVK, DM, JPM and MS. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BMI | = Body mass index |
| BSA | = Body surface area |
| CASPAR | = Classification Criteria for Psoriatic Arthritis |
| CI | = Confidence interval |
| DMARD | = Disease-modifying antirheumatic drug |
| FAS | = Full analysis set |
| FFbH | = Hannover Functional Ability Questionnaire |
| GRAPPA | = Group for Research and Assessment of Psoriasis and Psoriatic Arthritis |
| HAQ | = Health Assessment Questionnaire |
| LEI | = Leeds Enthesitis Index |
| LOCF | = Last observation carried forward |
| MDA | = Minimal disease activity |
| PASS | = Patient-acceptable symptom state |
| PDE4 | = Phosphodiesterase 4 |
| PhGA | = Physician’s Global Assessment |
| PPQ | = Patient Preference Questionnaire |
| PsA | = Psoriatic arthritis |
| PsAID-9 | = 9-item Psoriatic Arthritis Impact of Disease |
| PtGA | = Patient Global Assessment |
| SAE | = Serious treatment-emergent adverse event |
| SAS | = Safety analysis set |
| SD | = Standard deviation |
| SmPC | = Summary of product characteristics |
| SJC | = Swollen joint count |
| TEAE | = Treatment-emergent adverse event |
| TJC | = Tender joint count |
| VAS | = Visual analog scale |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the institutional review board IRB/IEC OF Freiburger ethic-komission International 016/1084.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed written consent was obtained from each patient prior to any study-related procedure.
AVAILABILITY OF DATA AND MATERIAL
All the data and supporting information is provided within the article. Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
CONFLICTS OF INTEREST
FB: AbbVie, Chugai, Janssen, Pfizer, Roche – grant/research support; AbbVie, Biotest, BMS, Celgene Corporation, Chugai, Eli Lilly, Genzyme, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, UCB – speakers bureau; AbbVie, Biotest, Boehringer Ingelheim, Celgene Corporation, Chugai, Eli Lilly, Genzyme, Janssen, Novartis, Pfizer, Roche, UCB – consultant.
JW: Abbott, BMS, MSD, Pfizer, UCB – grant/research support and consultant.
MF: AbbVie, Amgen, Celltrion, Galapagos, Novartis, Pfizer, UCB.
RvK: AbbVie, ALK Scherax, Almirall Hermal, Amgen, Beiersdorf Dermo Medical, Biofrontera, Biogen, BMS, Boehringer Ingelheim, Celgene, DermaPharm, Foamix, Galderma, Gilead, Hexal, Janssen-Cilag, LEO Pharma, Lilly, Meda, Medac, Menlo, MSD, Novartis, Dr. R. Pfleger, Pfizer, Regeneron, Sanofi, Stallergenes, Stiefel GSK, Tigercut, UCB.
DM: Amgen GmbH – former employee.
JPM and MS: Amgen GmbH – employee and stockholder.
FB: AbbVie, Chugai, Janssen, Pfizer, Roche – grant/research support; AbbVie, Biotest, BMS, Celgene Corporation, Chugai, Eli Lilly, Genzyme, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, UCB – speakers bureau; AbbVie, Biotest, Boehringer Ingelheim, Celgene Corporation, Chugai, Eli Lilly, Genzyme, Janssen, Novartis, Pfizer, Roche, UCB – consultant.
JW: Abbott, BMS, MSD, Pfizer, UCB – grant/research support and consultant.
MF: AbbVie, Amgen, Celltrion, Galapagos, Novartis, Pfizer, UCB.
RvK: AbbVie, ALK Scherax, Almirall Hermal, Amgen, Beiersdorf Dermo Medical, Biofrontera, Biogen, BMS, Boehringer Ingelheim, Celgene, DermaPharm, Foamix, Galderma, Gilead, Hexal, Janssen-Cilag, LEO Pharma, Lilly, Meda, Medac, Menlo, MSD, Novartis, Dr. R. Pfleger, Pfizer, Regeneron, Sanofi, Stallergenes, Stiefel GSK, Tigercut, UCB.
DM: Amgen GmbH – former employee.
JPM and MS: Amgen GmbH – employee and stockholder.
ACKNOWLEDGEMENTS
Writing support was sponsored by Amgen Inc. and provided by Rebecca Lane, PhD, of Peloton Advantage, LLC, an OPEN Health company.


